CH3OCH3 Lewis Structure, Molecular Geometry, Hybridization, And Polari…
페이지 정보
작성자 Hannah 작성일 24-11-25 21:07 조회 2 댓글 0본문
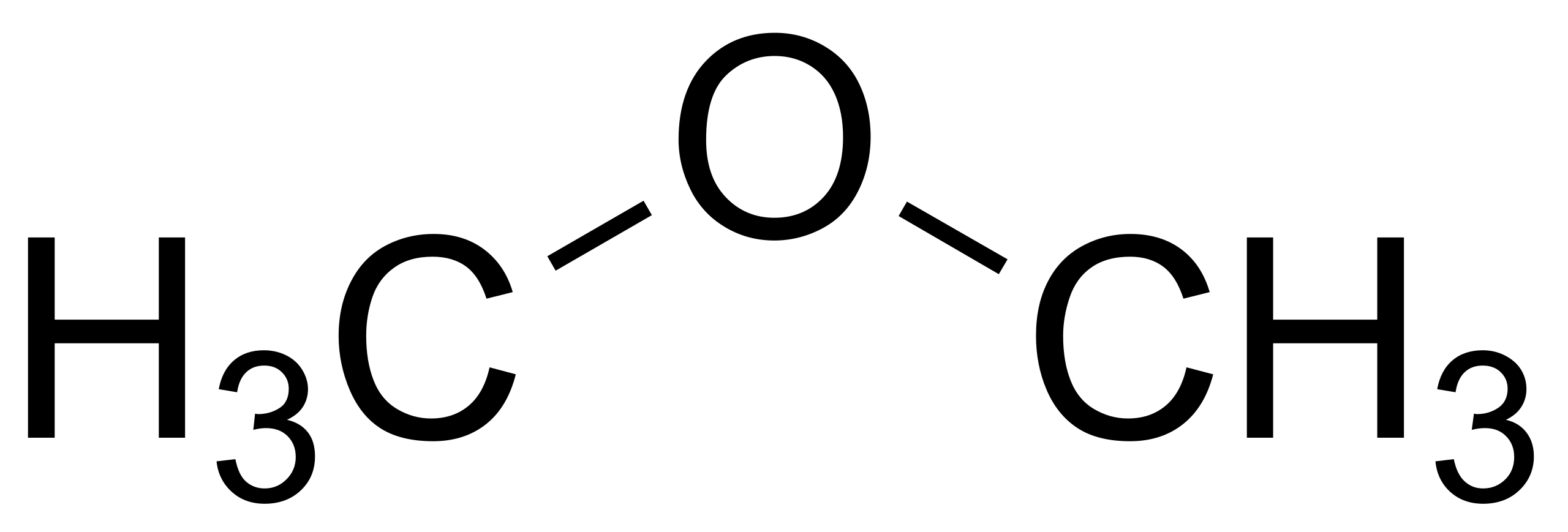
If we have a look at natural compounds like CH3OCH3, we have now covalent bonds formed between carbon and hydrogen and likewise carbon and oxygen. If we would like to explain this phenomenon of bond formation, we need to take the assistance of a model. Here we use the hybridization mannequin. Orbital hybridization refers to the combination and mixing of atomic orbitals to form hybrid orbitals. The atomic orbitals should be of the identical atom and bear equivalent energies. 2. Non-toxic and Colorless: When burnt, DME doesn't launch any hazardous pollutants and is non-toxic. Its adaptability is additionally increased because it has no coloration or smell. 3. Excessive Energy Density: The high energy density of DME makes it a fascinating fuel option for varied purposes. 1. Various Gas: DME has drawn curiosity as a cleaner substitute for LPG (liquefied petroleum gas) and diesel.
Though DME can be produced from biomass, methanol, and fossil fuels, the probably feedstock of selection for big-scale DME manufacturing in the United States is natural gas. DME could be produced immediately from synthesis gas produced from natural fuel, coal, or biomass. It can be produced not directly from methanol through a dehydration reaction. Transferring the mouse pointer over or close to a point will display the coordinates of the point. The number of digits proven don't reflect the uncertainty of the worth. Click in or tab to the plot space to enable keyboard commands. Additonal code used was developed at NIST: plot-knowledge.js. Kennedy, R.M.; Sagenkahn, M.; Aston, J.G., The heat capability and entropy, heats of fusion and vaporization, and the vapor strain of dimethyl ether. The density of gaseous dimethyl ether, J. Am. The National Institute of Requirements and Technology (NIST) makes use of its finest efforts to ship a top quality copy of the Database and to confirm that the information contained therein have been selected on the basis of sound scientific judgment. Nevertheless, NIST makes no warranties to that effect, and NIST shall not be liable for any damage that may consequence from errors or omissions within the Database. Customer support for NIST Commonplace Reference Knowledge products.
Data compiled by: NIST Mass Spectrometry Knowledge Middle, William E.S. Secretary of Commerce on behalf of the U.S.A. Pilcher, G.; Pell, A.S.; Coleman, D.J., Measurements of heats of combustion by flame calorimetry. Chao J., Thermodynamic properties of key organic oxygen compounds in the carbon vary C1 to C4. Half 2. Perfect fuel properties, J. Phys. Handi M.A., Molecular spectroscopy. The WEEL information for dimethyl ether (DME) was originally established in 1996 and updated in 2010. Literature searches to identify new toxicity data had been performed in November 2020 and evaluated by the WEEL Revisions Subcommittee. No new research or knowledge relevant to the WEEL information were identified. 200,000 ppm. In repeated inhalation studies of up to 2 years within the rat, DME was not carcinogenic and produced minimal toxicity at 25,000 and 10,000 ppm: the NOAEC was 2000 ppm. 5000 ppm. DME was not mutagenic in vitro or in vivo. The primary discovering with inhalation exposure was a reversible CNS depression. It was recognized that biodiesel is a gas that appropriates for compression ignition (diesel) engines, which is produced from biological sources reminiscent of fatty oils of vegetable or animal fats. DME fuel common rail system. Effect of injection timing on performance and emission. The studies of DME-biodiesel blends and the impact of EGR software on the toxicity of particles, performance, and engine emission were investigated by Sun et al.24,25 recently. The highlighted outcomes reveal that the addition of biodiesel will increase the entire particle quantity, the peak of particle quantity concentration, and the particle dimension corresponding to the peak. Furthermore, gasoline blends with biodiesel mass proportion ≤15% can stop abrasion and leakage within the engine, however there is no such thing as a apparent increase in both particle emissions and the potential for particle toxicity. In the meantime, the EGR affects the engine’s combustion by changing the chemical reaction, the O2 contents, and the particular heat capacities of the concentrations within the cylinders.
4. Refrigeration: It could also be utilized as a refrigerant in the cooling sector, especially in certain heat pump applications. 1. Methanol Dehydration: The primary technique is drying methanol (CH3OH) over an alumina-based strong acid catalyst to create DME and water. 2. Syngas Conversion: Another methodology contains turning syngas, a mix of hydrogen and carbon monoxide, into methanol and dehydrating it to create DME. Because of its adaptability and environmental advantages, the demand for DME has elevated worldwide. Either way, we are able to conclude that the oxygen atom has sp3 hybridization. What will we imply by Polarity? Polarity is the concept or subject in chemistry where we speak about charge distribution inside a molecule of a compound. We know that dipole second is used to measure electronegativity that is defined to be the diploma to which any atomic aspect can attain electrons.
댓글목록 0
등록된 댓글이 없습니다.

